FDA
-

How FDA Ramped up Medical Device Reviews
Following the onset of COVID-19, one office within the Food and Drug Administration leveraged new processes and developed innovative tools to support increased public health demands in regulating medical devices, leaders explained during FDA’s regulatory conference with industry this month.
7m read -

How FDA Ramped Up COVID-19 Treatment Approvals
When COVID-19 was declared a national emergency, the Food and Drug Administration had to act quickly on its oversight responsibilities around the sudden need for new testing, equipment and, ultimately, vaccines.
7m read -

Change Management Key to Transforming the Federal Workforce
As agencies modernize their IT infrastructure to remain competitive in the digital ecosystem, leaders are spearheading change management to ensure their workforce is up to speed.
7m read -

FDA Calls to Protect Drug Supply Chain During Pandemic
The Food and Drug Administration is advising industry partners on new guidance for identifying and tracing prescription drugs as they move through the distribution supply chain to ensure availability of critical drugs during the pandemic, Leigh Verbois, director of FDA’s Office of Drug Security, Integrity and Response, told GovernmentCIO Media & Research.
7m read -

COVID-19 Has Reshaped Federal Health IT Security
Changes brought by COVID-19 have produced a corresponding shift in federal cybersecurity posture, particularly among agencies responsible for overseeing health care and medical IT systems.
7m read -

FDA Tackles Training, Recruiting in Next Phase of Data Strategy
The Food and Drug Administration is breaking down silos, spearheading new data sharing efforts and upskilling its workforce under its data strategy to turn data into a “strategic asset” and drive new efficiencies.
7m read -

The New Age of Tobacco Product Application, Review Processes
The Food and Drug Administration’s Center for Tobacco Products is leveraging emerging technologies to drive new efficiencies across its premarket review process, the center’s Office of Science Director Matt Holman said during an FDA meeting last week.
7m read -

Pandemic Data of Vaping Use Shows Impact on Youth
The COVID-19 pandemic has had varying impacts on tobacco and vaping use among youths and adults, but did not have any impact on users’ reported intention to quit, according to recent data from the Food and Drug Administration.
7m read -

HHS Leaders Say Emerging Technologies Making Health Data Actionable
Emerging technologies and advances in infrastructure are making data across federal health agencies more actionable.
7m read -

FDA’s FY 2022 Budget Calls for Additional Funding to Strengthen IT Infrastructure
The Food and Drug Administration is requesting additional funding in its fiscal year 2022 budget to bolster data modernization, secure and strengthen supply chain, transition to cloud and develop new technology solutions.
7m read -
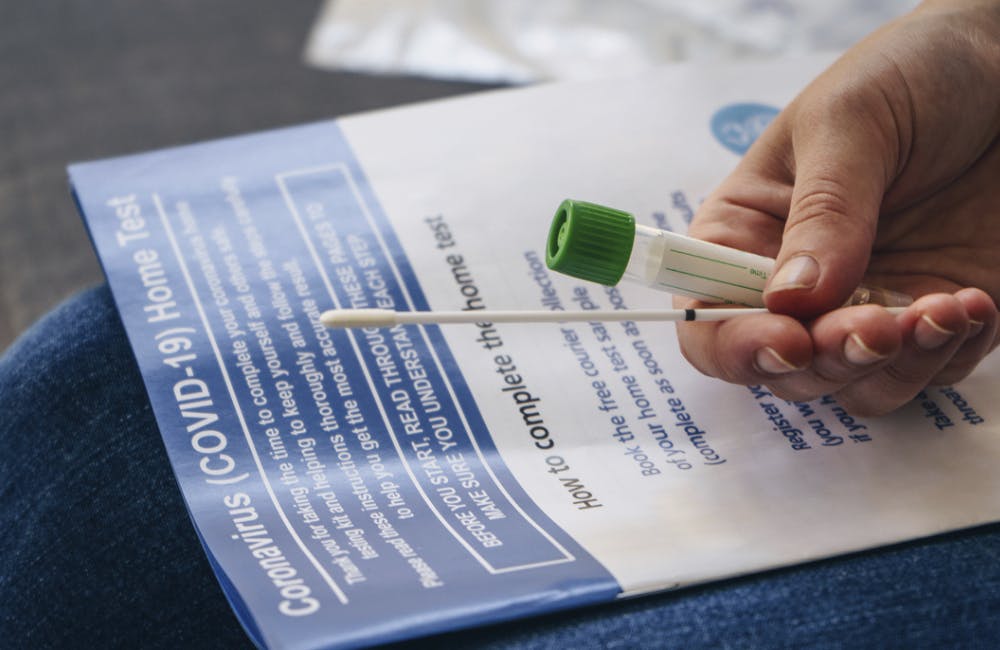
The Future of At-Home COVID-19 Testing
HHS’ at-home and self-testing services could help the public maintain health safety amid reopening plans.
7m read -

Tech’s Outlook to Advance New, Generic Drug Development
Offices within the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) are developing new tools to advance drug development and evaluation processes of generic and new medicines.
7m read














